Background: In the current era of acute myeloid leukemia (AML) management, hypomethylating agents (HMAs) remain as the backbone of combination regimens for front-line treatment of elderly or unfit patients (pts) and/or as salvage therapy in relapsed/refractory pts. Data on biological predictors of HMA response are limited. Our previous work explored cytidine deaminase (CDA), known to inactivate HMAs, and nucleophosmin 1 (NPM1), determined by core pathway analysis to indirectly influence CDA expression. No clear correlation was identified in pt samples between CDA protein expression and NPM1 status or response, and pharmacogenomic analysis showed no CDA single nucleotide polymorphisms were predictive of response to HMAs. We aimed to expand prior findings using RNA sequencing (RNA seq) and gene set enrichment analysis (GSEA) to identify gene signatures predictive of HMA response and propose potential therapeutic targets.
Methods: AML pts with banked samples who received frontline, bridging, or salvage HMA-based therapy between January 2014 to December 2018 were reviewed. Responses following at least 2 cycles of HMA were categorized as complete response (CR), CRi (CR with incomplete hematologic recovery), morphologic leukemia-free state (MLFS), complete hematologic response (CHR), or refractory. Pts were categorized as responders (CR, CRi, MLFS, CHR) or non-responders (refractory). Tumor cells were purified using immunomagnetic selection from bone marrow aspirates collected at diagnosis (dx). RNA seq was performed on 20 available pt tumor samples. Unsupervised clustering was performed and GSEA was completed to compare identified clusters using a false discovery rate (FDR) cut-off of less than 25%. GSEA-derived gene pathway scores were assessed by Wilcoxon rank-sum test. To evaluate AML cell lines to explore the non-responder phenotype, viability of AML cell lines was assessed following treatment with HMA azacitidine (Aza) and MS177 (a proteolysis targeting chimeric EZH2 degrader, also known to degrade MYC). Immunoblotting was used to assay proteolysis targeting chimeric (PROTAC)-induced MYC degradation.
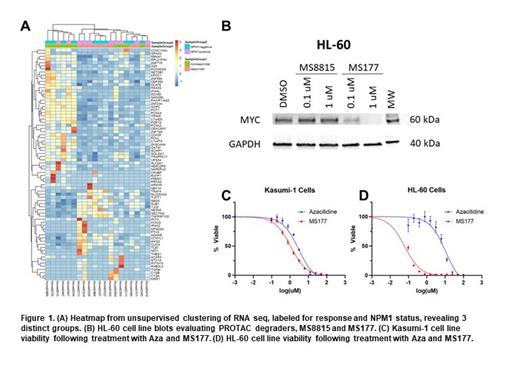
Results: Unsupervised clustering of RNA seq from 20 tumor samples at dx, labeled for response and NPM1 status, revealed 3 distinct groups: a non-responder group, a responder group, and a mixed response group ( Figure 1A). GSEA comparing the non-responder group to the responder group revealed several enriched genes in the non-responder group, including MYC targets (FDR <0.001), E2F targets (FDR <0.001), and G2M checkpoint (FDR=0.244). Upregulation of MYC targets was confirmed by enrichment profiling. Application of enrichment pathway scores revealed significant differences between non-responders and responders for E2F targets (p=0.026), G2M checkpoint (p=0.026), and MYC targets (p=0.0022). To explore MYC as a therapeutic target, we assayed MYC degradation in leukemia cells treated with two PROTAC degraders. The PROTAC MS177 induced marked MYC degradation in HL-60 cells ( Figure 1B). Cytotoxicity assays showed that in Kasumi-1 cells, the 50% inhibitory concentrations (IC50) for Aza and MS177 were 2.9 µM (responder phenotype) and 1.1 µM, respectively ( Figure 1C); in HL-60 cells the IC50 for Aza and MS177 were 10.9 µM (non-responder phenotype) and 0.06 µM, respectively ( Figure 1D). MS177 also showed efficacy in cytotoxicity assays using pt derived tumor samples.
Conclusions: Using tumor samples from AML pts receiving HMAs, we identified a non-responder gene phenotype enriched with MYC upregulation. GSEA-derived pathway scoring differentiated non-responders from responders in our dataset. To further evaluate whether the enrichment pathway score is predictive for response across other AML cohorts, we are applying enrichment scores to publicly available AML datasets. With MYC upregulation identified as a potential driver of HMA failure, MYC degradation or inhibitory targets are recognized as potential therapeutic targets for pts with a non-responder gene signature. We established the HL-60 AML cell line, found to be less sensitive to Aza than the Kasumi-1 cell line, demonstrated greater sensitivity to MYC degradation compared to Kasumi-1 cells and may reflect the non-responder phenotype. Further in vitro efforts are ongoing to evaluate MYC targets for the HMA non-responder phenotype.
Disclosures
Ragon:Pfizer: Other: Advisory board; Astellas: Other: Advisory board; Genentech: Other: Advisory Board. Jin:Cullgen, Inc: Current Employment, Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees, Other: Co-Founder, Research Funding; Onsero Therapeutics: Current Employment, Membership on an entity's Board of Directors or advisory committees, Other: Scientific Cofounder; EpiCypher, Inc: Current Employment, Other: Consultant ; Accent Therapeutics, Inc: Other: Consultant ; Tavotek Biotherapeutics, Inc: Current Employment, Other: Consultant ; Celgene Corporation: Research Funding; Levo Therapeutics, Inc: Research Funding; Cullinan Oncology, Inc: Research Funding. Park:Epizyme: Membership on an entity's Board of Directors or advisory committees; Morphosys: Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Research Funding; BMS: Research Funding. Grunwald:AbbVie, Agios/Servier, Amgen, Astellas Pharma, Blueprint Medicines, Bristol Myers Squibb, Cardinal Health, CTI BioPharma/Sobi, Daiichi Sankyo, Gamida Cell, Genentech, Gilead Sciences, GSK/Sierra Oncology, Incyte Corporation, Invitae, Jazz Pharmaceuticals: Consultancy; Incyte Corporation, Janssen: Research Funding; Medtronic: Current equity holder in publicly-traded company; Karius, Novartis, Ono Pharmaceutical, Pfizer, Pharmacosmos, Premier, Stemline Therapeutics: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal